Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Clarifying Questions:
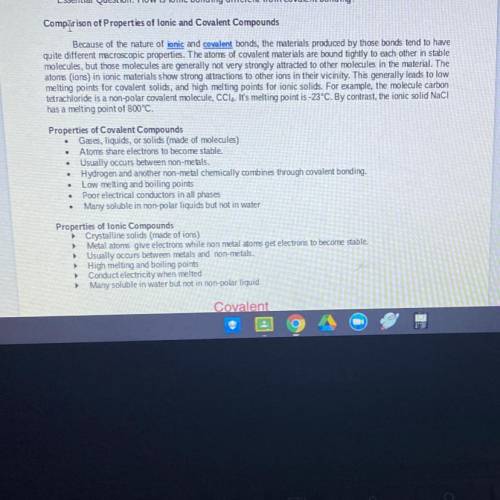
1. Why do solid covalent compounds ( molecules) have low melting points?
Questions

Mathematics, 07.01.2020 10:31


Biology, 07.01.2020 10:31



Mathematics, 07.01.2020 10:31


Computers and Technology, 07.01.2020 10:31

History, 07.01.2020 10:31

Mathematics, 07.01.2020 10:31

Computers and Technology, 07.01.2020 10:31



Biology, 07.01.2020 10:31

Mathematics, 07.01.2020 10:31

Mathematics, 07.01.2020 10:31




Mathematics, 07.01.2020 10:31