
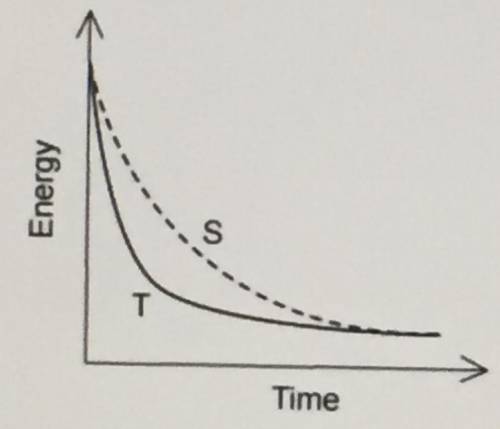
A student was investigating the rate of reaction between a solid base and a solution of
sulfuric acid. Two experiments were performed, S and T, in which the mass of the
reaction flask was recorded as shown in the graph.
Which of the following changes could explain the difference in results between S and T?
A ) The sulfuric acid is less concentrated in T.
B ) The sulfuric acid is more concentrated in T.
C ) A higher temperature is used in S.
D) Larger sized particles are used in T.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3


Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1
You know the right answer?
A student was investigating the rate of reaction between a solid base and a solution of
sulfuric ac...
Questions

Biology, 16.10.2020 16:01

English, 16.10.2020 16:01




Mathematics, 16.10.2020 16:01

Chemistry, 16.10.2020 16:01


English, 16.10.2020 16:01




Mathematics, 16.10.2020 16:01




History, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


History, 16.10.2020 16:01



