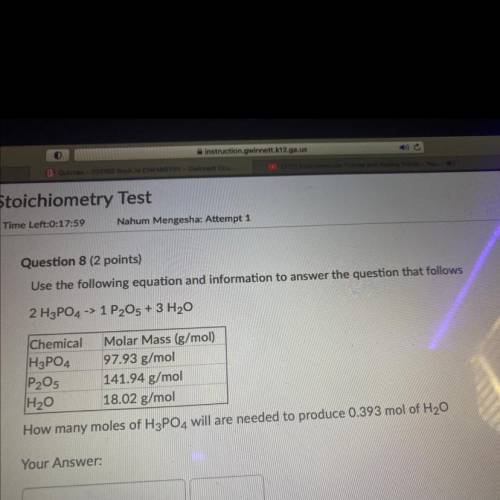
Question 8 (2 points)
3
Use the following equation and information to answer the question tha...

Chemistry, 23.02.2021 22:10 msmaporter
Question 8 (2 points)
3
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P2O5 + 3 H2O
6
9
Chemical Molar Mass (g/mol)
H3PO4 97.93 g/mol
P2O5 141.94 g/mol
H20 18.02 g/mol
How many moles of H3PO4 will are needed to produce 0.393 mol of H20
Your
Answer
units

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Questions


Mathematics, 12.07.2019 15:20

Physics, 12.07.2019 15:20





History, 12.07.2019 15:20

History, 12.07.2019 15:20

Mathematics, 12.07.2019 15:20

English, 12.07.2019 15:20

Social Studies, 12.07.2019 15:20

History, 12.07.2019 15:20


History, 12.07.2019 15:20



Computers and Technology, 12.07.2019 15:20


Computers and Technology, 12.07.2019 15:20



