
Chemistry, 23.02.2021 21:10 deanlmartin
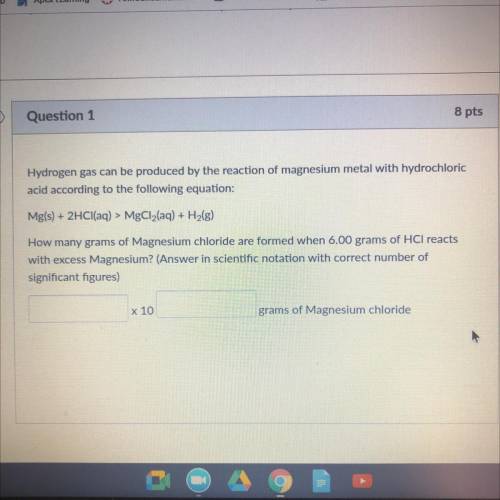
Hydrogen gas can be produced by the reaction of magnesium metal WICH
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are Irmed when 6.00 grams of HCl reacts
with excess Magnesium? (Answer in scientific notation with correct number of
significant figures)
x 10
grams of Magnesium chloride

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
Hydrogen gas can be produced by the reaction of magnesium metal WICH
acid according to the followin...
Questions

Mathematics, 18.08.2019 22:30


Physics, 18.08.2019 22:30

History, 18.08.2019 22:30


Mathematics, 18.08.2019 22:30


Mathematics, 18.08.2019 22:30




Mathematics, 18.08.2019 22:30

Mathematics, 18.08.2019 22:30

Chemistry, 18.08.2019 22:30


Mathematics, 18.08.2019 22:30




Mathematics, 18.08.2019 22:30



