
Chemistry, 23.02.2021 14:20 sadieanngraham15
determine the rate of reaction at 10 seconds when : a) concentration of acid is used at 1.0M b) concentration of acid is used at 1.5M
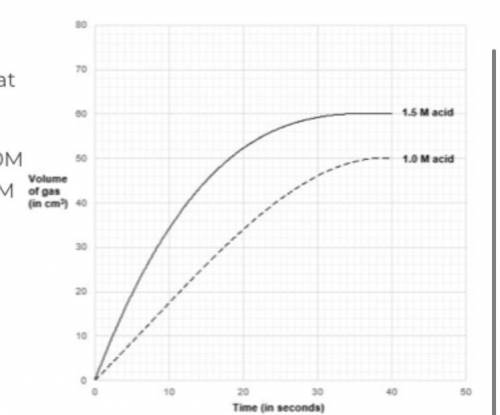

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
determine the rate of reaction at 10 seconds when : a) concentration of acid is used at 1.0M b) conc...
Questions

Mathematics, 04.12.2020 20:30



History, 04.12.2020 20:30

Arts, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30


Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Chemistry, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Computers and Technology, 04.12.2020 20:30

Social Studies, 04.12.2020 20:30


English, 04.12.2020 20:30


Geography, 04.12.2020 20:30



