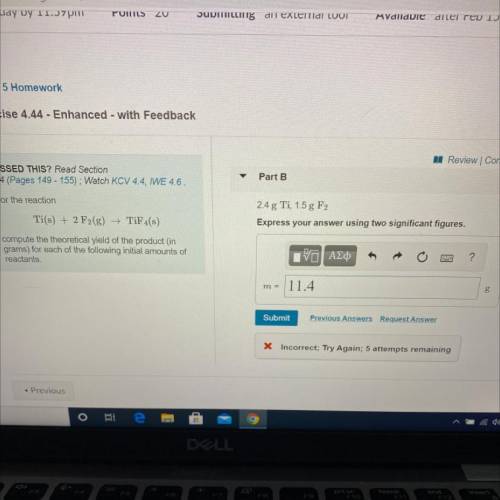
compute the theoretical yield of the product (in

Chemistry, 23.02.2021 09:10 marissagirl9893
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
grams) for each of the following initial amounts of
reactants.
2.4 g Ti, 1.5 g F2
Express your answer using two significant figures.
please help! will give brainliest.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
compute the theoretical yield of the product (in
Questions

Mathematics, 01.07.2019 11:30




Mathematics, 01.07.2019 11:30



Mathematics, 01.07.2019 11:30



Mathematics, 01.07.2019 11:30

English, 01.07.2019 11:30

Biology, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30


Mathematics, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30

Health, 01.07.2019 11:30



