
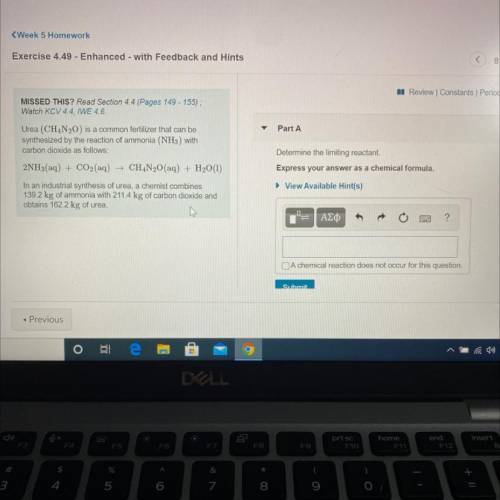
Urea (CH4N20) is a common fertilizer that can be
synthesized by the reaction of ammonia (NH3) with
carbon dioxide as follows:
Determine the limiting reactant.
Express your answer as a chemical formula.
2NH3(aq) + CO2(aq) + CH4N20(aq) + H2O(1)
In an industrial synthesis of urea, a chemist combines
139.2 kg of ammonia with 211.4 kg of carbon dioxide and
obtains 162.2 kg of urea.
Determine the limiting reactant

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Urea (CH4N20) is a common fertilizer that can be
synthesized by the reaction of ammonia (NH3) with...
Questions




Social Studies, 26.05.2021 18:30


Health, 26.05.2021 18:30



English, 26.05.2021 18:30

Mathematics, 26.05.2021 18:30


English, 26.05.2021 18:30

English, 26.05.2021 18:30

Mathematics, 26.05.2021 18:30




Mathematics, 26.05.2021 18:30


Mathematics, 26.05.2021 18:30



