
Chemistry, 23.02.2021 07:20 titofigueroa777
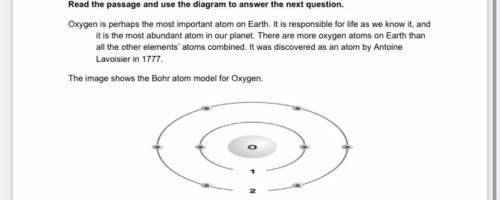
Which of the following electronic transitions in the oxygen atom will result in light emission?
A. n i= 1⟶nf = 2
B. n i= 1⟶nf =3
C. n i =3⟶n f =2
D. n i =2⟶n f =3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
Which of the following electronic transitions in the oxygen atom will result in light emission?
A....
Questions


Mathematics, 13.08.2020 06:01


English, 13.08.2020 06:01





Mathematics, 13.08.2020 06:01

Biology, 13.08.2020 06:01








Advanced Placement (AP), 13.08.2020 06:01

Mathematics, 13.08.2020 06:01



