
Chemistry, 23.02.2021 04:10 mimiloveyuhh
2C3H6O + 8O2 --> 6CO2 + 6H2O
The combustion reaction for propanol is given above. How many moles of water are produced if 0.15 moles of propanol (C3H6O) reacts with an excess amount oxygen gas?
Please try to show work if possible!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
2C3H6O + 8O2 --> 6CO2 + 6H2O
The combustion reaction for propanol is given above. How many moles...
Questions

History, 21.07.2019 03:00

History, 21.07.2019 03:00

Health, 21.07.2019 03:00

Biology, 21.07.2019 03:00

History, 21.07.2019 03:00




Social Studies, 21.07.2019 03:00

Social Studies, 21.07.2019 03:00




History, 21.07.2019 03:00

Business, 21.07.2019 03:00


Business, 21.07.2019 03:00

Biology, 21.07.2019 03:00

History, 21.07.2019 03:00

Mathematics, 21.07.2019 03:00

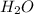 will be produced from 0.15 moles of propanol.
will be produced from 0.15 moles of propanol.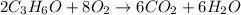
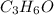 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and 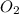 is the excess reagent.
is the excess reagent.
 will produce=
will produce=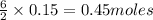 of
of 

