
Chemistry, 23.02.2021 04:10 tammycute01
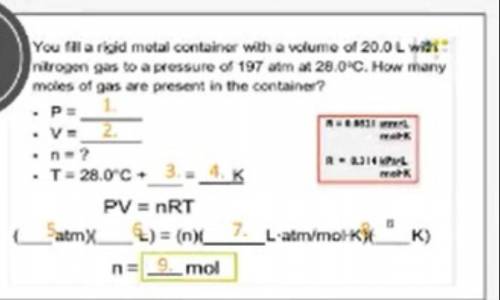
a rigid metal container has a volume of 20.0 L with nitrogen gas to a pressure of 197atm at 28.0 C. How many moles of gas are present in the container

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2
You know the right answer?
a rigid metal container has a volume of 20.0 L with nitrogen gas to a pressure of 197atm at 28.0 C....
Questions

Social Studies, 02.08.2019 16:00


Physics, 02.08.2019 16:00


Computers and Technology, 02.08.2019 16:00

Mathematics, 02.08.2019 16:00

Computers and Technology, 02.08.2019 16:00



Biology, 02.08.2019 16:00


Computers and Technology, 02.08.2019 16:00



Computers and Technology, 02.08.2019 16:00

Mathematics, 02.08.2019 16:00






