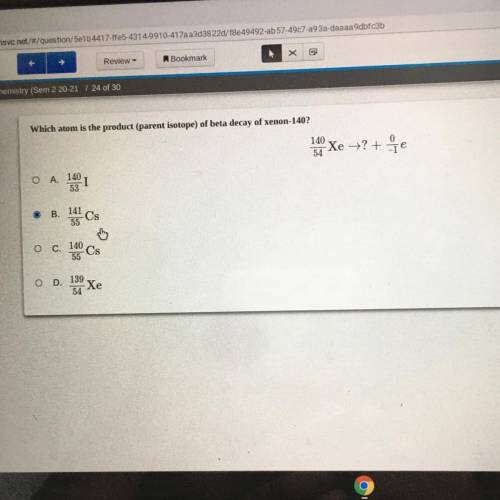
Which atom is the product (parent isotope) of beta decay of xenon-140?
140
Xe = ?+7e
54...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
Questions

Physics, 08.03.2021 16:20

Business, 08.03.2021 16:20

Geography, 08.03.2021 16:20



Mathematics, 08.03.2021 16:20

Physics, 08.03.2021 16:20



Mathematics, 08.03.2021 16:20

Mathematics, 08.03.2021 16:20


Chemistry, 08.03.2021 16:20


Mathematics, 08.03.2021 16:20

Chemistry, 08.03.2021 16:20

Computers and Technology, 08.03.2021 16:30

Mathematics, 08.03.2021 16:30

Mathematics, 08.03.2021 16:30