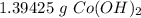Chemistry, 22.02.2021 23:30 Uhmjujiooo4220
Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2NaCl + Co(OH)₂
If you react 10.0 mL of 1.5 M CoCl₂ with plenty of NaOH, how many grams of Co(OH)₂ will precipitate out?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2Na...
CoCl₂ + 2NaOH → 2Na...
Questions

English, 27.10.2020 01:10

SAT, 27.10.2020 01:10

History, 27.10.2020 01:10



Mathematics, 27.10.2020 01:10


English, 27.10.2020 01:10

Mathematics, 27.10.2020 01:10

Computers and Technology, 27.10.2020 01:10


Mathematics, 27.10.2020 01:10

Mathematics, 27.10.2020 01:10


Health, 27.10.2020 01:10



Chemistry, 27.10.2020 01:10


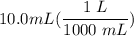 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 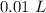 [DA] Find moles of CoCl₂ [Molarity]:
[DA] Find moles of CoCl₂ [Molarity]: 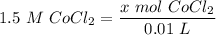 [DA] Solve for x [Multiplication Property of Equality]:
[DA] Solve for x [Multiplication Property of Equality]: 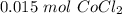 [DA] Set up [Reaction Stoich]:
[DA] Set up [Reaction Stoich]: 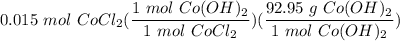 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: