Chemistry, 22.02.2021 19:50 villarrealc1987
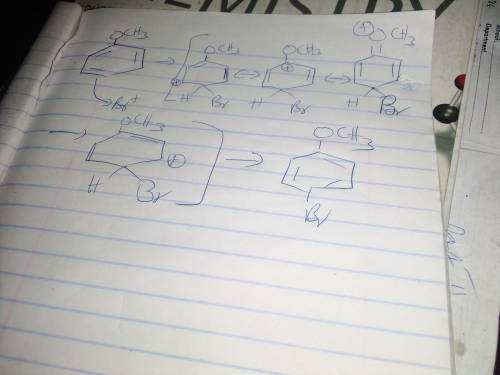
During electrophilic aromatic substitution, a resonance-stabilized cation intermediate is formed. Groups, already present on the benzene ring, that direct ortho/para further stabilize this intermediate by participating in the resonance delocalization of the positive charge. Assume that the following group is present on a benzene ring at position 1 and that you are brominating the ring at positon 4. In the box below draw the structure of the resonance contributor that shows this group actively participating in the charge delocalization. -OCH3 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
During electrophilic aromatic substitution, a resonance-stabilized cation intermediate is formed. Gr...
Questions

English, 24.04.2020 21:52




History, 24.04.2020 21:53



Mathematics, 24.04.2020 21:53


Mathematics, 24.04.2020 21:53

Chemistry, 24.04.2020 21:53





Biology, 24.04.2020 21:53



Chemistry, 24.04.2020 21:53