
Chemistry, 22.02.2021 19:30 tejalawatson91
Which of the following are correct for zero-order reactions?
A. A higher concentration of reactants will not increase the reaction rate.
B. The units for the rate constant and the rate of reaction are the same.
C. The rate of reaction does not equal the rate constant.
D. The concentration of the reactants changes nonlinearly.
E. A zero-order reaction slows down as the reaction proceeds.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Which of the following are correct for zero-order reactions?
A. A higher concentration of reactants...
Questions

Mathematics, 15.09.2021 14:00


Health, 15.09.2021 14:00





English, 15.09.2021 14:00

Biology, 15.09.2021 14:00


History, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00


Social Studies, 15.09.2021 14:00


Mathematics, 15.09.2021 14:00



Mathematics, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00

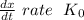 doesn't depend on reaction rate, a higher reaction rate does not intensify the reaction.
doesn't depend on reaction rate, a higher reaction rate does not intensify the reaction.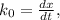 the created based and the reaction rate is about the same.
the created based and the reaction rate is about the same.

