
Chemistry, 30.09.2019 03:30 ConfusedJuliana
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775 ml of water. calculate the following:
a) moles of k2so4
b)millimoles of k2so4
c)molarity of k2so4, k+, so4(2-)
d)ppm of k2so4
e)%(w/v) k2so4
f)pk+
g)pso4(2-)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775...
Questions


Mathematics, 29.07.2019 08:00






Biology, 29.07.2019 08:00


Business, 29.07.2019 08:00

Mathematics, 29.07.2019 08:00









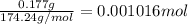
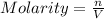
![[K_2SO_4]=\frac{0.001016 mol}{0.775 L}=0.001311 mol/L](/tpl/images/0275/4296/7b54d.png)
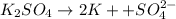
![[K^+]=2\times [K_2SO_4]=2\times 0.001311 mol/L=0.002622 mol/L](/tpl/images/0275/4296/87b7a.png)
![[SO_4^{2-}]=1\times [K_2SO_4]=1\times 0.001311 mol/L=0.001311 mol/L](/tpl/images/0275/4296/fd0e3.png)
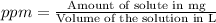
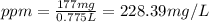
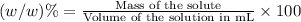
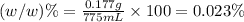
![pK^=-\log[K^+]](/tpl/images/0275/4296/a9ce4.png)
![pK^+=-\log[0.002622 M]=2.58](/tpl/images/0275/4296/0acce.png)
![pSO_4^{2-}=-\log[SO_4^{2-}]](/tpl/images/0275/4296/bd352.png)
![pK^+=-\log[0.001311 M]=2.88](/tpl/images/0275/4296/30697.png)


