
Chemistry, 22.02.2021 18:30 lovelybear2354
You will be provided with the AH values for each equation. These values are state dependent,
meaning that liquid water and steam will have different values. You will need to multiply the
AH, value for each compound by the number of moles of that compound in the balanced
chemical equation, as each value of AH, is for 1 mole of compound. Find the enthalpy of
reaction for the following equation:
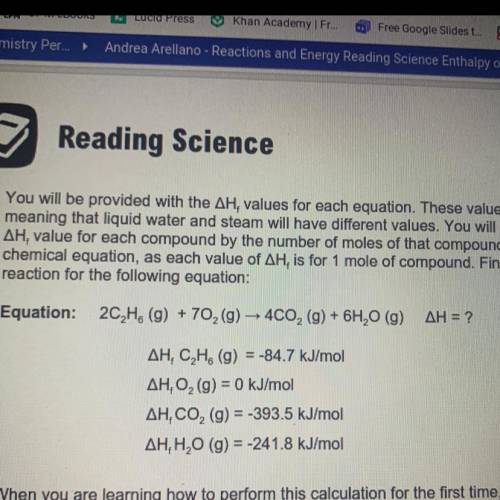

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
You will be provided with the AH values for each equation. These values are state dependent,
meanin...
Questions


Mathematics, 02.07.2019 10:30






Mathematics, 02.07.2019 10:30

Chemistry, 02.07.2019 10:30


Biology, 02.07.2019 10:30

Biology, 02.07.2019 10:30


Mathematics, 02.07.2019 10:30

English, 02.07.2019 10:30


Mathematics, 02.07.2019 10:30

Mathematics, 02.07.2019 10:30

Mathematics, 02.07.2019 10:30



