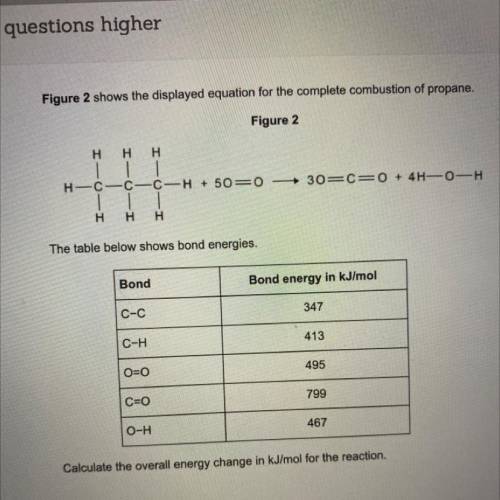
Figure 2 shows the displayed equation for the complete combustion of propane.
Figure 2
Η Η Η<...

Chemistry, 22.02.2021 18:20 ngmasuku3115
Figure 2 shows the displayed equation for the complete combustion of propane.
Figure 2
Η Η Η
30=CEO + 4H-0-H
H-C-C-C-H + 50=0
| |
H Η Η
The table below shows bond energies.
Bond
Bond energy in kJ/mol
C-C
347
C-H
413
O=0
495
C=0
799
O-H
467
Calculate the overall energy change in kJ/mol for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identify the balanced chemical equation that represents a decomposition reaction. p4 + 3o2 ⟶ p2o3 2fe(oh)3 ⟶ 2feo3 + h2o cuso4 ⟶ cuo + 2so3 2fe(oh)3 ⟶ fe2o3 + 3h2o
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

You know the right answer?
Questions

English, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00





Mathematics, 03.03.2021 01:00


History, 03.03.2021 01:00


Biology, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00



Mathematics, 03.03.2021 01:00

English, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00




