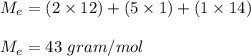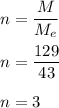Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
a compound has a molar mass of 129 g/mol if its empirical formula is C2H5N then what is the molecula...
Questions



Mathematics, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40




Computers and Technology, 09.03.2021 16:40

French, 09.03.2021 16:40

Social Studies, 09.03.2021 16:40

History, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40





Mathematics, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40

Mathematics, 09.03.2021 16:40