Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

You know the right answer?
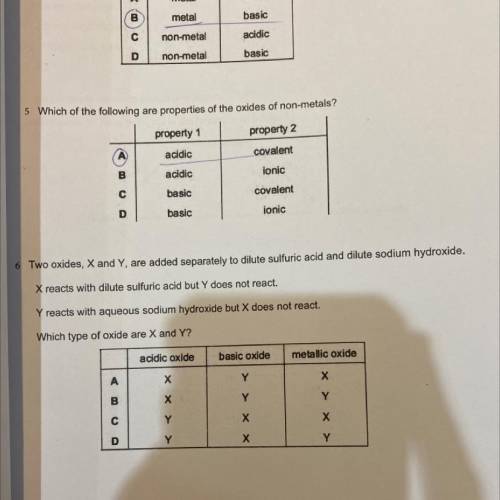
two oxides, X and Y are added seperately to dilute sulfuric acid and dilute sodium hydroxide. X reac...
Questions

Geography, 20.10.2019 16:30


Mathematics, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

History, 20.10.2019 16:30


Biology, 20.10.2019 16:30

Chemistry, 20.10.2019 16:30



Health, 20.10.2019 16:30


Chemistry, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30




Mathematics, 20.10.2019 16:30