
Chemistry, 21.02.2021 18:00 donaldwilliams31
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aqueous solution of H2S04. (IF = 96500C, Volume at s. t.p = 22.4
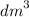

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aque...
Questions

Mathematics, 23.04.2020 17:47












Biology, 23.04.2020 17:48

History, 23.04.2020 17:48



English, 23.04.2020 17:48

English, 23.04.2020 17:48





