
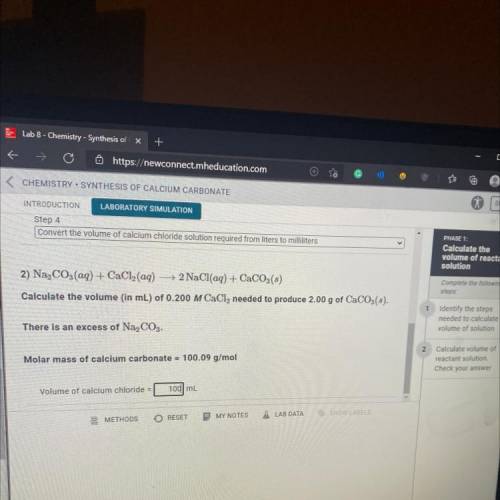
Convert the volume of calcium chloride solution required from liters to milliliters
2) Na, CO3(aq) + CaCl2(aq) + 2 NaCl(aq) + CaCO3(3)
Calculate the volume (in mL) of 0.200 M CaCl, needed to produce 2.00 g of CaCO3(s
There is an excess of Na2CO3.
Molar mass of calcium carbonate = 100.09 g/mol
mL
Volume of calcium chloride =
RESET
MY NOTES
A LAB DATA
METHODS

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

You know the right answer?
Convert the volume of calcium chloride solution required from liters to milliliters
2) Na, CO3(aq)...
Questions

History, 26.03.2020 05:03



Mathematics, 26.03.2020 05:03

Mathematics, 26.03.2020 05:03

Biology, 26.03.2020 05:03

Mathematics, 26.03.2020 05:04



Biology, 26.03.2020 05:04

History, 26.03.2020 05:04


Computers and Technology, 26.03.2020 05:04





English, 26.03.2020 05:05

Chemistry, 26.03.2020 05:05



