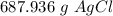Chemistry, 20.02.2021 05:00 Hcalhoun21
How many grams of silver chloride can be produced by reacting excess silver nitrate with 2.4 moles of zinc chloride? AgNO3 + ZnCl2 AgCl + Zn(NO3)2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
How many grams of silver chloride can be produced by reacting excess silver nitrate with 2.4 moles o...
Questions






History, 05.12.2020 06:00

Business, 05.12.2020 06:00

History, 05.12.2020 06:00

Mathematics, 05.12.2020 06:00


Mathematics, 05.12.2020 06:00

Mathematics, 05.12.2020 06:00

Mathematics, 05.12.2020 06:00







Geography, 05.12.2020 06:00

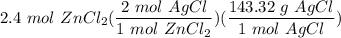 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: