⚠️URGENT PLZ HELP TIMED TEST⚠️
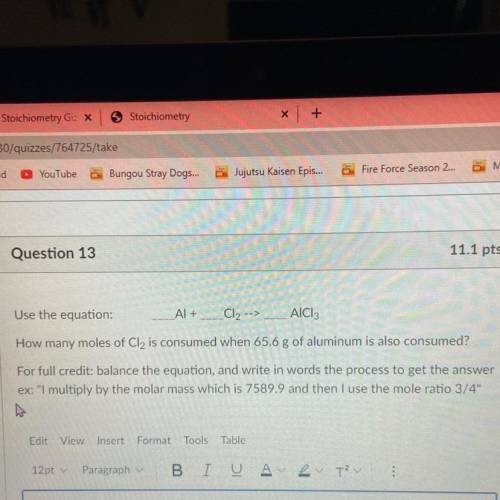
Use the equation:
Al +
Cl2 -->
AICI:
---<...

Chemistry, 19.02.2021 23:40 ImmortalEnigmaYT
⚠️URGENT PLZ HELP TIMED TEST⚠️
Use the equation:
Al +
Cl2 -->
AICI:
---
How many moles of Cl2 is consumed when 65.6 g of aluminum is also consumed?
For full credit: balance the equation, and write in words the process to get the answer
ex: "I multiply by the molar mass which is 7589.9 and then I use the mole ratio 3/4"

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
Questions

Advanced Placement (AP), 07.12.2020 22:30

Business, 07.12.2020 22:30

Chemistry, 07.12.2020 22:30

English, 07.12.2020 22:30




English, 07.12.2020 22:30






Mathematics, 07.12.2020 22:30


Social Studies, 07.12.2020 22:30



Spanish, 07.12.2020 22:30



