
Chemistry, 19.02.2021 22:30 yaneiryx5476
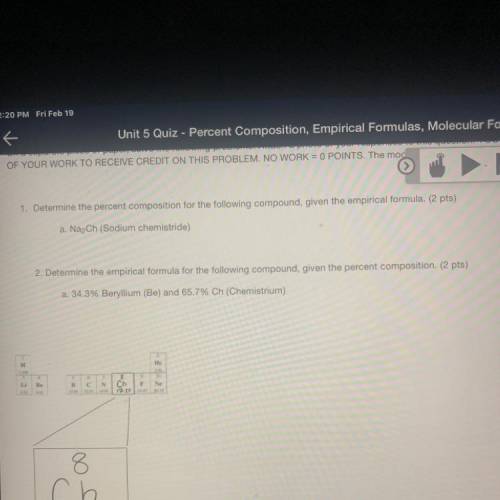
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts)
a. Na2Ch (Sodium chemistride)
2. Determine the empirical formula for the following compound, given the percent composition. (2 pts)
a. 34.3% Beryllium (Be) and 65.7% Ch (Chemistrium)
H
Не
Be
B
c С
N
Ch F Ne
17.250 30
8
ch
Chemistrium
17.25

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Two friends at different locations want to communicate with each other by sending low energy signals. which of the following methods can they use to communicate? a) produce x-rays using colliding electrons and send them to radios, which capture sound b) send messages using infrared radiation, which travel in the form of waves c) send radio waves through intervening media like radio and television d) produce sound waves using microwaves from heated objects
Answers: 2

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts...
Questions

Biology, 02.11.2019 00:31



Chemistry, 02.11.2019 00:31


Mathematics, 02.11.2019 00:31


Mathematics, 02.11.2019 00:31


History, 02.11.2019 00:31

Mathematics, 02.11.2019 00:31

History, 02.11.2019 00:31


Mathematics, 02.11.2019 00:31


Mathematics, 02.11.2019 00:31






