
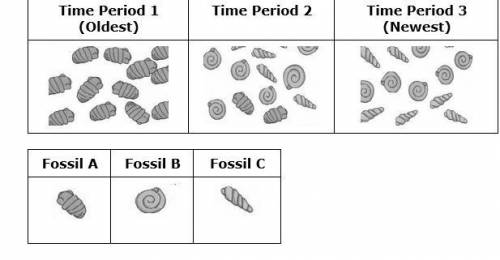
Use the information and the tables to answer the following question.
The tables show fossil evidence found in one area during three different time periods.
The environment during Time period 1 was very hot and rainy. Over time, the environment became much drier.
What can you conclude from this information?
A. Fossil C survived best in a rainy environment because there is a large population of them in Time period 3.
B. Fossil B struggled to survive in a dry environment because there are none of Fossil B in Time Period 1.
C. Fossil C was best adapted for a dry environment because their population increased in Time Period 3.
D. Fossil A was better adapted to a dry environment because there is a larger population of them in Time Period 1.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
The electron configurations of two different atoms are shown below. each yellow electron has a charge of 1−, and the net charge of each nucleus is shown. these atoms will combine with bond. a. an ionic b. a positive c. a negative d. a covalent plzzz mee with !
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Use the information and the tables to answer the following question.
The tables show fossil evidenc...
Questions

Geography, 24.06.2019 16:00




Mathematics, 24.06.2019 16:00

History, 24.06.2019 16:00




English, 24.06.2019 16:00










Mathematics, 24.06.2019 16:00



