
Chemistry, 19.02.2021 18:50 Jsmooth8928
1 pt
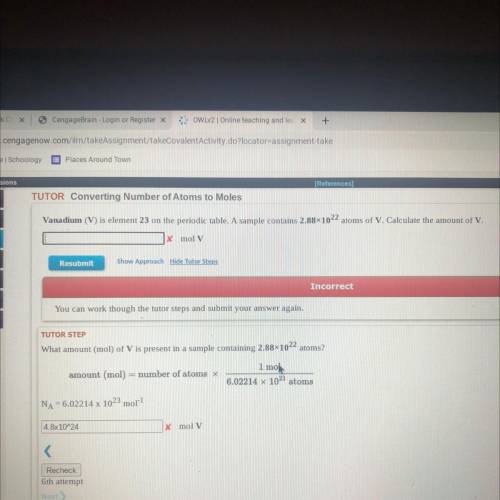
Vanadium (V) is element 23 on the periodic table. A sample contains 2.88x1022 atoms of V. Calculate the amount of V.
1 pt
x mol V
1 pt
Resubmit
Show Approach Hide Tutor Steps

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
1 pt
Vanadium (V) is element 23 on the periodic table. A sample contains 2.88x1022 atoms of V. Calc...
Questions

Chemistry, 10.06.2020 07:57




Mathematics, 10.06.2020 07:57

Business, 10.06.2020 07:57

Mathematics, 10.06.2020 07:57

English, 10.06.2020 07:57


English, 10.06.2020 07:57


English, 10.06.2020 07:57


Mathematics, 10.06.2020 07:57






Social Studies, 10.06.2020 07:57



