
Chemistry, 19.02.2021 06:10 yasameenakbari
What is the molecular formula for a compound that is 44.87% potassium, 36.7% oxygen, 18.0% sulfur and a molecular mass of 696g.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
What is the molecular formula for a compound that is 44.87% potassium, 36.7%
oxygen, 18.0% sulfur a...
Questions








Chemistry, 10.02.2020 22:19




History, 10.02.2020 22:19

Social Studies, 10.02.2020 22:19




History, 10.02.2020 22:19

Geography, 10.02.2020 22:20


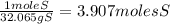
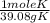 = 7.99117 mole K
= 7.99117 mole K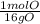 = 15.9654 mole O
= 15.9654 mole O

