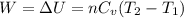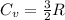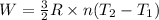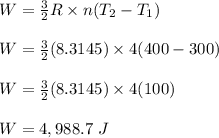Chemistry, 19.02.2021 05:00 helpmewithmath70
4 moles of monoatomic ideal gas is compressed adiabatically causing the temperature to increase from 300 K to 400 K. Calculate the work done on the gas in units of Joules (if the answer is negative, be sure to enter a negative sign in your answer).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1


Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
4 moles of monoatomic ideal gas is compressed adiabatically causing the temperature to increase from...
Questions

English, 06.05.2020 00:37

Mathematics, 06.05.2020 00:37

Mathematics, 06.05.2020 00:37

Mathematics, 06.05.2020 00:37




Computers and Technology, 06.05.2020 00:37


Computers and Technology, 06.05.2020 00:37

Mathematics, 06.05.2020 00:37

Chemistry, 06.05.2020 00:37


Mathematics, 06.05.2020 00:37


Mathematics, 06.05.2020 00:37

Mathematics, 06.05.2020 00:37


Arts, 06.05.2020 00:37