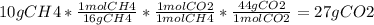Chemistry, 18.02.2021 23:30 aletadaboss
20 POINTS When methane, CH4, is combusted, and produces carbon dioxide, CO2, according to the unbalanced equation CH4 +02 —>CO2 + H2O Write the balanced equation for this reaction, and explain how it is possible for 10 grams of methane fuel to burn in and emit 27 grams of carbon dioxide. Discuss whether or not this reaction obeys the law of conservation of mass.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
20 POINTS When methane, CH4, is combusted, and produces carbon dioxide, CO2, according to the unbala...
Questions



Mathematics, 13.10.2020 09:01


Mathematics, 13.10.2020 09:01

Geography, 13.10.2020 09:01

History, 13.10.2020 09:01


History, 13.10.2020 09:01


Mathematics, 13.10.2020 09:01

Mathematics, 13.10.2020 09:01



History, 13.10.2020 09:01

Mathematics, 13.10.2020 09:01

Mathematics, 13.10.2020 09:01


Mathematics, 13.10.2020 09:01