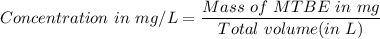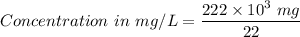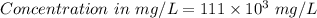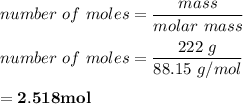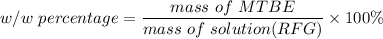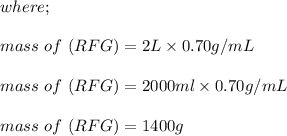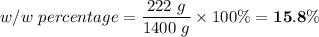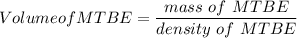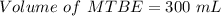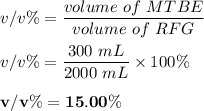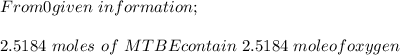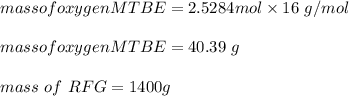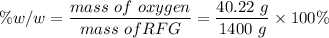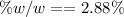Chemistry, 18.02.2021 20:40 QueenBlumple4443
222 g of MTBE (CO(CH3)4) are added to gasoline, resulting in a total volume of 2 L of reformulated gas (RFG). Assume that the density of RFG is 0.70 g/mL, and the density of MTBE is 0.74 g/mL. For a-d, determine the concentration of MTBE in the RFG in the following units?
a. mg/L
b. moles/L
c. % (w/w)
d. % (v/v)
e. What is the % (w/w) oxygen in the RFG due to MTBE?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
222 g of MTBE (CO(CH3)4) are added to gasoline, resulting in a total volume of 2 L of reformulated g...
Questions

Mathematics, 08.10.2019 01:00

English, 08.10.2019 01:00


Mathematics, 08.10.2019 01:00

Mathematics, 08.10.2019 01:00

Chemistry, 08.10.2019 01:00





Physics, 08.10.2019 01:00




Chemistry, 08.10.2019 01:00


Mathematics, 08.10.2019 01:00

Mathematics, 08.10.2019 01:00

Geography, 08.10.2019 01:00

Mathematics, 08.10.2019 01:00