1.61 x 104 molecules
Od
2.26 x 1029 molecules
Oe
1.77 x 1029 molecules
Ques...

1.61 x 104 molecules
Od
2.26 x 1029 molecules
Oe
1.77 x 1029 molecules
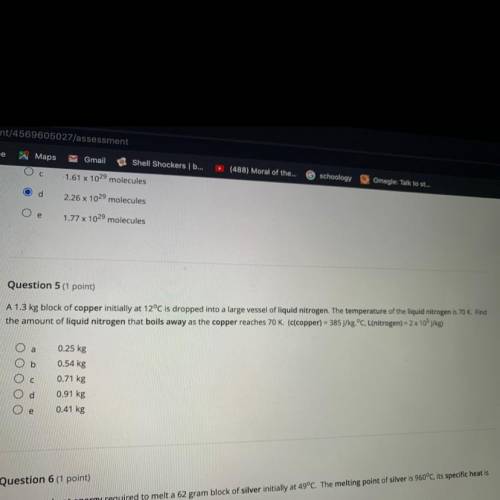
Question 5 (1 point)
A 1.3 kg block of copper initially at 12°C is dropped into a large vessel of liquid nitrogen. The temperature of the liquid nitrogen is 70 K. Find
the amount of liquid nitrogen that boils away as the copper reaches 70 K. (c(copper) = 385 J/kg.°C, L(nitrogen) = 2 x 105 j/kg

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Questions



English, 21.05.2020 01:57

Mathematics, 21.05.2020 01:57

History, 21.05.2020 01:57




History, 21.05.2020 01:57


History, 21.05.2020 01:57

History, 21.05.2020 01:57



Mathematics, 21.05.2020 01:57

Physics, 21.05.2020 01:57

Engineering, 21.05.2020 01:57


Spanish, 21.05.2020 01:57



