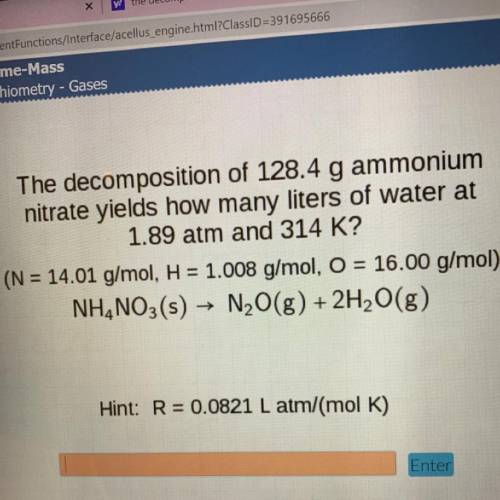
The decomposition of 128.4 g ammonium
nitrate yields how many liters of water at
1.89 atm and...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Questions

English, 04.02.2020 01:44

Social Studies, 04.02.2020 01:44


Mathematics, 04.02.2020 01:44


History, 04.02.2020 01:44



Mathematics, 04.02.2020 01:44


History, 04.02.2020 01:44



Mathematics, 04.02.2020 01:44

Mathematics, 04.02.2020 01:44



Social Studies, 04.02.2020 01:44

Mathematics, 04.02.2020 01:44

Geography, 04.02.2020 01:44