
Chemistry, 11.10.2019 18:50 cadeedmiston
Using the periodic table, explain the difference between hydrogen, deuterium, and tritium - i. e. hydrogen-1, hydrogen-2, and hydrogen-3.
a) allotropes vary in their number of protons - so they contain 1, 2, and 3 protons, respectively.
b) the atoms deviate in their number of photons - so they contain 1, 2, and 3 photons, respectively.
c) isotopes differ only in their number of neutrons - so they contain 0, 1, and 2 neutrons, respectively.
d) these configurations diverge in their number of electrons - so they contain 1, 2, and 3 electrons, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Using the periodic table, explain the difference between hydrogen, deuterium, and tritium - i. e. hy...
Questions



Spanish, 18.09.2021 14:00



English, 18.09.2021 14:00


Mathematics, 18.09.2021 14:00


Arts, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00

Social Studies, 18.09.2021 14:00


History, 18.09.2021 14:00


Engineering, 18.09.2021 14:00

Biology, 18.09.2021 14:00

World Languages, 18.09.2021 14:00

History, 18.09.2021 14:00

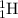 , deuterium
, deuterium 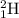 , and tritium
, and tritium  .
.

