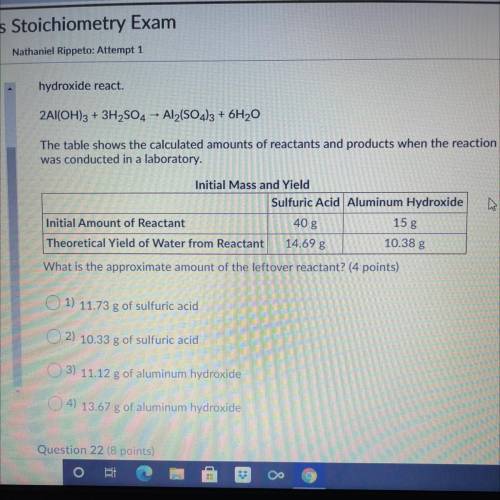
hydroxide react.


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
hydroxide react.
Questions




Chemistry, 30.05.2020 01:58



History, 30.05.2020 01:58

English, 30.05.2020 01:58

English, 30.05.2020 01:58


Mathematics, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58

English, 30.05.2020 01:58

Mathematics, 30.05.2020 01:58




Mathematics, 30.05.2020 01:58