
Chemistry, 17.02.2021 21:40 emblemhacks
A. Calculate the percentage yield for SiO2 for trial 1. Also, determine the leftover reactant for the trial. Show your Work. b. Based on the percentage yield in trial 2, explain what ratio of reactants is more efficient for the given reaction.
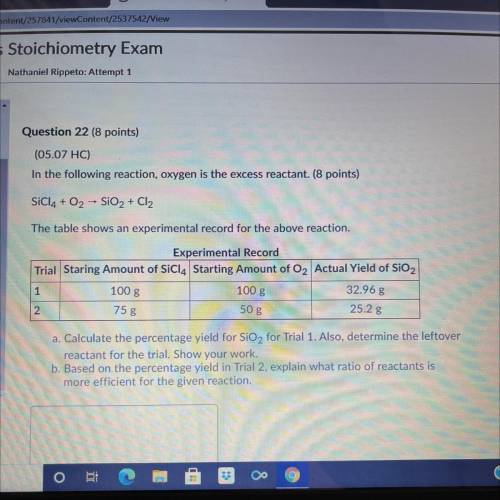

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

You know the right answer?
A. Calculate the percentage yield for SiO2 for trial 1. Also, determine the leftover reactant for th...
Questions

Biology, 31.07.2019 10:50

History, 31.07.2019 10:50

Biology, 31.07.2019 10:50

History, 31.07.2019 10:50

Chemistry, 31.07.2019 10:50


Social Studies, 31.07.2019 11:00

History, 31.07.2019 11:00


History, 31.07.2019 11:00


English, 31.07.2019 11:00

History, 31.07.2019 11:00



Social Studies, 31.07.2019 11:00


Mathematics, 31.07.2019 11:00

Social Studies, 31.07.2019 11:00

Social Studies, 31.07.2019 11:00



