
Chemistry, 17.02.2021 17:10 brookeguilford
HELP One use of CaCl 2 to salt roads in the winter. The equation that describes the change in freezing point of a solvent due to a solute is T f =i x K f x molality. How much would the freezing point decrease if a 3.23 molal (m) solution were achieved? (K f =1.86^ C/(mol/kg) for water and i = 3 for CaCl 2 )
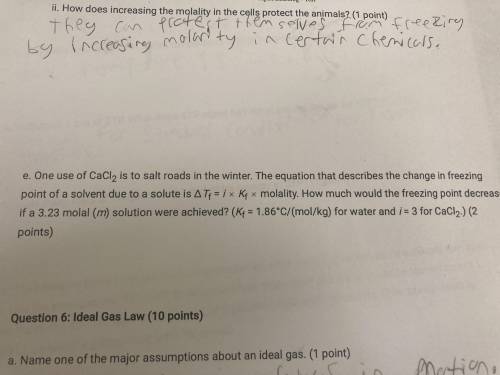

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1


Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
HELP
One use of CaCl 2 to salt roads in the winter. The equation that describes the change in freez...
Questions





Social Studies, 07.12.2021 23:50

English, 07.12.2021 23:50



Biology, 07.12.2021 23:50


Mathematics, 07.12.2021 23:50





Mathematics, 07.12.2021 23:50


Mathematics, 07.12.2021 23:50

Mathematics, 07.12.2021 23:50

Mathematics, 07.12.2021 23:50



