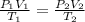Chemistry, 17.02.2021 14:10 jfitness11
A gas in a piston starts out with a volume of 156 mL, a temperature of 28.1° C, and a pressure of 1.12 atm. If it ends with a volume of 312 mL and a temperature of 87.2° C, what is the new pressure?


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
A gas in a piston starts out with a volume of 156 mL, a temperature of 28.1° C, and a pressure of 1....
Questions




Social Studies, 29.06.2019 06:30

Biology, 29.06.2019 06:30

History, 29.06.2019 06:30




Geography, 29.06.2019 06:30


Mathematics, 29.06.2019 06:30

History, 29.06.2019 06:30

History, 29.06.2019 06:30

English, 29.06.2019 06:30


Mathematics, 29.06.2019 06:30


Computers and Technology, 29.06.2019 06:30

English, 29.06.2019 06:30