
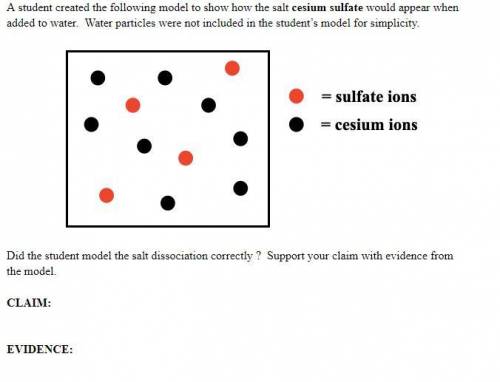
A student created the following model to show how the salt cesium sulfate would appear when added to water. Water particles were not included in the student’s model for simplicity. Did the student model the salt dissociation correctly ? Support your claim with evidence from the model.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
A student created the following model to show how the salt cesium sulfate would appear when added to...
Questions






Computers and Technology, 02.03.2020 18:29










History, 02.03.2020 18:29






