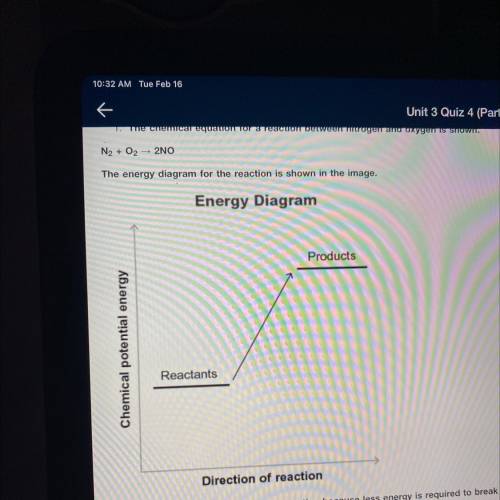
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
T...

Chemistry, 16.02.2021 21:50 brookie125
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
Then energy diagram for the reaction is shown in the image.
A) energy is absorbed, less energy, break bonds, form new bonds.
B) energy is released, more energy, break bonds, form new bonds
C) Energy is absorbed, the bond energy of the reactant is higher than the bond energy of the products
D) Energy is released, the bond energy of the reactant is lower than the bond energy of the products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
Questions

English, 18.02.2021 05:00

Mathematics, 18.02.2021 05:00




Computers and Technology, 18.02.2021 05:00

Mathematics, 18.02.2021 05:00






Law, 18.02.2021 05:00


Biology, 18.02.2021 05:00


Mathematics, 18.02.2021 05:00


Mathematics, 18.02.2021 05:00



