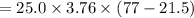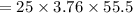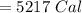Chemistry, 16.02.2021 01:00 brattymoo1009
The specific heat of a metal is 3.76 cal/g°C. How much heat energy will it take to heat a 25.0 g cylinder of the metal from 21.5°C to 77.0°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
The specific heat of a metal is 3.76 cal/g°C. How much heat energy will it take to heat a 25.0 g cyl...
Questions

Mathematics, 07.12.2020 02:50

Mathematics, 07.12.2020 02:50


Mathematics, 07.12.2020 02:50


English, 07.12.2020 02:50

History, 07.12.2020 02:50


Mathematics, 07.12.2020 02:50



History, 07.12.2020 02:50



Advanced Placement (AP), 07.12.2020 02:50


Mathematics, 07.12.2020 02:50


Mathematics, 07.12.2020 02:50

Mathematics, 07.12.2020 02:50