
Chemistry, 15.02.2021 22:10 marandahuber
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to produced Ammonium. Which of the reacted will be in excess and by how many moles?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to p...
Questions



Mathematics, 02.12.2019 03:31





Mathematics, 02.12.2019 03:31

English, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Social Studies, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Mathematics, 02.12.2019 03:31

Chemistry, 02.12.2019 03:31



Mathematics, 02.12.2019 03:31

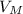 = 22, 4 l/mol
= 22, 4 l/mol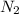 ) = 6,72
) = 6,72 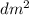 = 6,72 l
= 6,72 l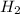 ) = 6,72
) = 6,72 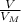
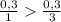 (we have more hydrogen than is need for the reaction)
(we have more hydrogen than is need for the reaction)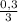 = 0,1 mol (
= 0,1 mol (

