Chemistry, 15.02.2021 20:20 princessroyal
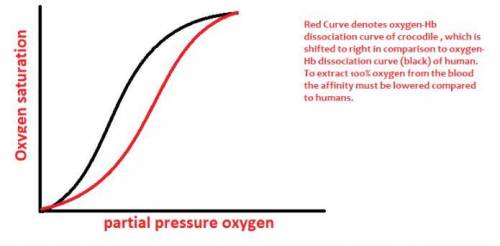
The crocodile which can remain underwater without breathing for up to an hour, drowns it air-breathing prey and then dines on them at its leisure. An adaptation that aids the crocodile in doing so is that it can utilize virtually 100% of the O2 in its blood whereas humans, for example, can extract only -65% of the O2 in their blood. (a) Draw an O2 binding curve comparing crocodile hemoglobin to that of normal hemoglobin. humne ih (b) Experiments have demonstrated that 2,3 bis-phosphoglycerate has no effect on the O2 binding of crocodile hemoglobin. However, it has been shown that 2 molecules of bicarbonate bind to deoxyhemoglobin tetramers but not to oxyhemoglobin tetramers of crocodile hemoglobin. Describe how bicarbonate might effect the O2 affinity of crocodile hemoglobin. Make sure to detail what regions of the hemoglobin tetramek might be bound by bicarbonate and how this would influence structural changes in the crocodile hemoglobin that would, in turn, affect O2 affinity. (c) Sketch the biochemical reactions in red blood cells that result in bicarbonate formation in venous blood. Write balanced equations and identify any enzymes that might be involved. If an enzyme is required, indicate which reaction is enzyme-catalyzed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 07:50
Many reactions take place in aqueous solution. when potential reactants are mixed, a reaction will occur if there is some driving force that favors the formation of products. it is often convenient to categorize reactions in terms of these driving forces: precipitate formation, in which an insoluble solid is formed, weak electrolyte formation, as in a neutralization reaction involving water, or transfer of electrons, as in a redox reaction. these reactions can be represented by full molecular equations, which contain all species in the reaction mixture, or by net ionic equations, which show only the species that actually undergo a change. the latter does not contain the spectator ions, which do not undergo a net change or do not take part in the reaction. part a when the following two solutions are mixed: k2co3(aq)+fe(no3)3(aq) the mixture contains the ions listed below. sort these species into spectator ions and ions that react. drag the appropriate items to their respective bins. view available hint(s) spectator ions ions that react part b what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.ba(oh)2(aq)+h2so4(aq)→ express your answer as a chemical equation. view available hint(s) nothing provide feedback
Answers: 3

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
The crocodile which can remain underwater without breathing for up to an hour, drowns it air-breathi...
Questions

English, 18.10.2020 15:01


Biology, 18.10.2020 15:01

Spanish, 18.10.2020 15:01

History, 18.10.2020 15:01


World Languages, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01


Social Studies, 18.10.2020 15:01


World Languages, 18.10.2020 15:01



Mathematics, 18.10.2020 15:01