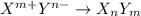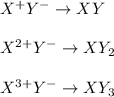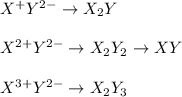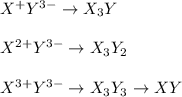Suppose that X represents an arbitrary cation and that Y represents an anionic species. Using the charges indicated in the superscript of X and Y, fill in the remaining blanks below by writing the appropriate subscript for each X and Y to balance the net charge on the ionic XaYb compound formed (where a and b represent positive, whole numbers of X and Y, respectively).
Y- Y2- Y3-
X+
X2+ XY2
X3+

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
Suppose that X represents an arbitrary cation and that Y represents an anionic species. Using the ch...
Questions

English, 09.11.2020 16:20







Mathematics, 09.11.2020 16:20


Mathematics, 09.11.2020 16:20


Computers and Technology, 09.11.2020 16:20

Spanish, 09.11.2020 16:20






Mathematics, 09.11.2020 16:20