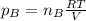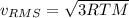Chemistry, 15.02.2021 19:40 mikehager4321
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Which of the two gases exerts the greater partial pressure?
b. The molecules or atoms of which gas have the greater average velocity?
c. The molecules of which gas have the greater average kinetic energy?
d . If a small hole were opened in the flask, which gas effuses more quickly?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Whi...
Questions




Mathematics, 08.06.2020 21:57


Biology, 08.06.2020 21:57

English, 08.06.2020 21:57

Mathematics, 08.06.2020 21:57

English, 08.06.2020 21:57

Mathematics, 08.06.2020 21:57







Mathematics, 08.06.2020 21:57

Mathematics, 08.06.2020 21:57