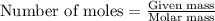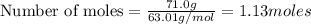Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles...
Questions


Biology, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Biology, 20.10.2019 15:30



Mathematics, 20.10.2019 15:30

History, 20.10.2019 15:30


Business, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

English, 20.10.2019 15:30






Mathematics, 20.10.2019 15:30

English, 20.10.2019 15:30

History, 20.10.2019 15:30

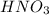 means 71.0 g of
means 71.0 g of