1.
Use the Rydberg equation to calculate the energy (in J) and then find the wavelength
in (n...

Chemistry, 13.02.2021 14:00 laurenrubin18
1.
Use the Rydberg equation to calculate the energy (in J) and then find the wavelength
in (nm) for the following transitions.
-18
AE = 2.179x10
a.
3-2.


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Questions





Mathematics, 27.04.2021 21:50

Chemistry, 27.04.2021 21:50

Computers and Technology, 27.04.2021 21:50

Mathematics, 27.04.2021 21:50





Mathematics, 27.04.2021 21:50

Biology, 27.04.2021 21:50

Mathematics, 27.04.2021 21:50

History, 27.04.2021 21:50

Mathematics, 27.04.2021 21:50

Mathematics, 27.04.2021 21:50

Mathematics, 27.04.2021 21:50

Mathematics, 27.04.2021 21:50

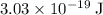 , which corresponds to a wavelength of approximately
, which corresponds to a wavelength of approximately 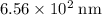 in vacuum.
in vacuum.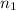 to
to 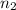 , the energy change would be:
, the energy change would be: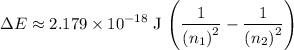 .
.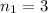 to
to 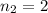 :
: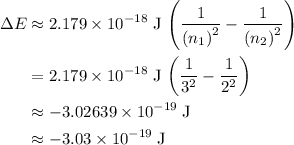 .
.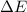 is negative because energy is released during this transition.
is negative because energy is released during this transition.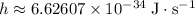 .Speed of light in vacuum:
.Speed of light in vacuum: 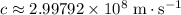 .
. of photons from this transition using the Planck-Einstein relation:
of photons from this transition using the Planck-Einstein relation: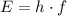 .
.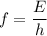 .
.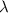 of these photos in vacuum:
of these photos in vacuum: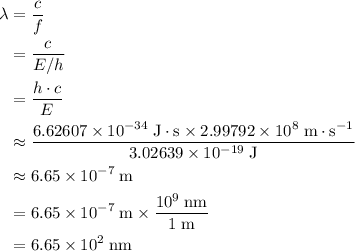 .
. 

