
Chemistry, 13.02.2021 04:40 latoyatuggle23
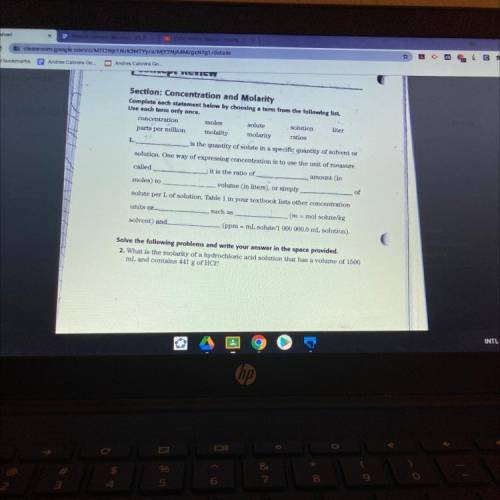
Section: Concentration and Molarity
Complete each statement below by choosing a term from the following list.
Use each term only once.
concentration
moles
solute solution liter
parts per million molality molarity ratios
1.
is the quantity of solute in a specific quantity of solvent or
solution. One way of expressing concentration is to use the unit of measure
called
it is the ratio of
amount in
moles) to
volume (in liters), or simply
of
solute per L of solution. Table 1 in your textbook lists other concentration
units or
such as
(m = mol solute/kg
solvent) and
(ppm = ml solute/1 000 000.0 mL solution).
nments
hent to Maureen
wankwa
Solve the following problems and write your answer in the space provided.
2. What is the molarity of a hydrochloric acid solution that has a volume of 1500
ml and contains 141 g of HCI?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

You know the right answer?
Section: Concentration and Molarity
Complete each statement below by choosing a term from the follo...
Questions

Mathematics, 27.09.2019 07:30




Mathematics, 27.09.2019 07:30


Chemistry, 27.09.2019 07:30




Mathematics, 27.09.2019 07:30

Health, 27.09.2019 07:30

Mathematics, 27.09.2019 07:30



History, 27.09.2019 07:30

Mathematics, 27.09.2019 07:30


Biology, 27.09.2019 07:30



