Consider the following reaction:
Cl2 + Na2S -> 2NaCl + S
The percent yield of sulfur...

Chemistry, 13.02.2021 04:20 yupthatsme2121
Consider the following reaction:
Cl2 + Na2S -> 2NaCl + S
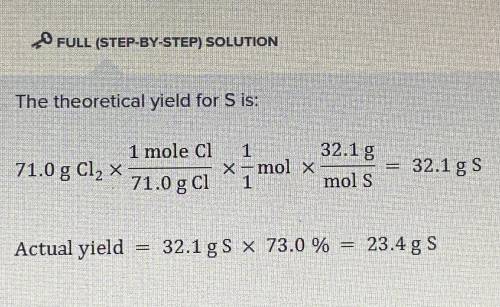
The percent yield of sulfur when 71.0 g of Cl2 is reacted in excess Na2S solution is 73.0 %.
What is the actual mass of sulfur yielded by this reaction?
(Molar mass of S = 32.1 g/mol, molar mass of Cl2 = 71.0 g/mol)
__ g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1


Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
Questions







Mathematics, 12.02.2020 05:01

Mathematics, 12.02.2020 05:01


Mathematics, 12.02.2020 05:01


Mathematics, 12.02.2020 05:01






English, 12.02.2020 05:01