Name of element ionized:

Chemistry, 12.02.2021 21:30 student0724
PLEASE HELP!
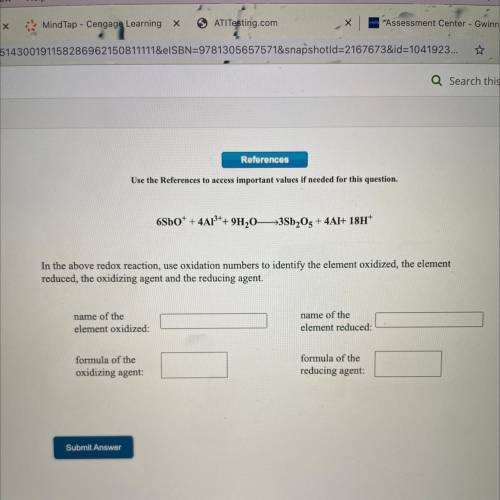
6SbO^+ + 4Al^3+ 9H2O -> 3Sb2O5 + 4Al + 18H^+
Name of element ionized:
Name of element reduced:
Formula of the oxidizing agent:
Formula of the reducing agent:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
PLEASE HELP!
6SbO^+ + 4Al^3+ 9H2O -> 3Sb2O5 + 4Al + 18H^+
Name of element ionized:
Name of element ionized:
Questions

Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40


Arts, 09.05.2021 03:40

Social Studies, 09.05.2021 03:40





Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40



Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40

Medicine, 09.05.2021 03:40

Computers and Technology, 09.05.2021 03:40

Computers and Technology, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40



