
Chemistry, 12.02.2021 19:30 edgartorres5123
Which is true about the compounds carbon monoxide (CO) and carbon dioxide (CO2)?
A. The compounds have the same properties because they contain the same elements.
B. The compounds have different properties because they are found in different amounts in the atmosphere.
C. The compounds have the same properties because they are both gases.
D. The compounds have different properties because they contain the same elements in different quantities.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
Which is true about the compounds carbon monoxide (CO) and carbon dioxide (CO2)?
A. The compounds h...
Questions


Computers and Technology, 29.10.2019 06:31



Mathematics, 29.10.2019 06:31

Physics, 29.10.2019 06:31




English, 29.10.2019 06:31


Mathematics, 29.10.2019 06:31

History, 29.10.2019 06:31

Mathematics, 29.10.2019 06:31




Physics, 29.10.2019 06:31


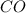 contains carbon and oxygen in the ratio of 3: 4 by mass whereas
contains carbon and oxygen in the ratio of 3: 4 by mass whereas 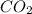 contains carbon and oxygen in the ratio of 3: 8 by mass.
contains carbon and oxygen in the ratio of 3: 8 by mass.

